Background: Multiple myeloma (MM) is a heterogeneous disease that may be evaluated by a broad array of imaging and laboratory techniques to measure disease activity and predict prognosis. FDG PET/CT has been shown to be predictive of patient outcomes throughout the disease course, with several studies demonstrating that PET/CT is an independent prognostic factor for progression free survival and overall survival (OS). We sought to corroborate these findings by specifically examining the prognostic impact of PET/CT in the post-transplant setting. We retrospectively analyzed PET/CT in MM patients after consolidation therapy with autologous stem cell transplant (ASCT) and correlated these findings with time to progression (TTP) and OS to assess the impact of day 100 PET/CT findings as an independent prognostic factor.
Methods: A retrospective cohort study was conducted on 231 myeloma patients who underwent ASCT between 2003-2016 and had a FDG PET/CT exam available for analysis at an average of day 104 post-transplant. We recorded the findings on PET/CT prior to transplant, where available. PET/CT without evidence of active/residual disease (PET-) was defined per IMWG guidelines (disappearance of every area of increased tracer uptake found on preceding PET/CT, or uptake < mediastinal blood pool, or decrease < surrounding normal tissue). PET/CT indicating active disease (PET+) was defined as any abnormal uptake or incompletely resolved uptake from previous exam. A Kaplan-Meier model was used to estimate median TTP and OS and the 2-sided log-rank test to compare groups. TTP and OS were calculated from the date of ASCT to the date of confirmed disease progression or death, respectively. Outcomes were then compared among patients with a PET/CT versus those without a PET/CT post-ASCT to assess for the presence of selection bias. A proportional hazards fit model was used for multivariate analysis.
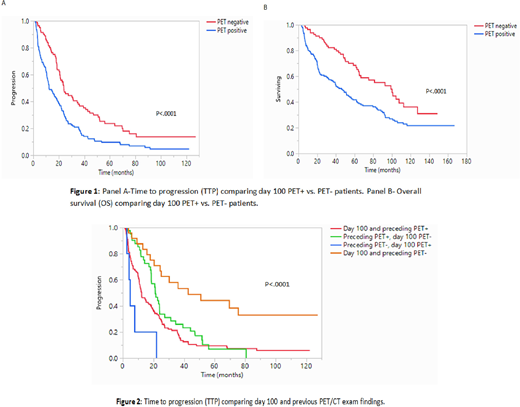
Results: The median length of follow-up for the entire cohort was 49 months (range: 43-58 mo), median TTP was 18.5 months (95% CI; 15.4-21.8), and median OS was 61.5 months (95% CI; 49-75). Among the entire cohort (n=231), 150 (65%) patients had a positive PET/CT exam at day 100 post-ASCT while 81 (35%) had a negative PET exam. There were 195 (84%) patients with a PET/CT exam prior to ASCT available for comparison, of which 161 (85%) were PET+ and 29 (15%) were PET-. Median time between PET/CT exams was 4.1 months (range 1.4-55). Median TTP among PET+ patients near day 100 was 12.4 months vs. 24 months PET- patients (p<.0001, Fig 1, panel A). Median OS in the PET+ group was 46 months compared to 99 months in the PET- group (p<.0001, Fig 1, panel B). Among patients with a prior PET/CT (195, 84%), median TTP was longer in patients with both exams negative compared to those who were day 100 PET- and previously PET+ (42.6 mo vs. 21.1 mo respectively; p<.0001). TTP was shorter in those who were day 100 PET+ and previously PET- compared to those with persistently PET+ on both exams (4.5 vs. 12.3 mo; p<.0001, Figure 2). Since PET/CT was not routinely ordered post ASCT, we also compared the outcomes between patients getting a PET/CT (n=231) and remaining patients undergoing ASCT (n=1764). Among patients with a day 100 PET/CT vs. those without a PET/CT exam, there was a slightly longer median TTP and OS in the group who did not have a post-ASCT PET/CT. Hazard ratios (HR) after adjusting for disease response (CR at day 100 vs. non-CR) were as follows: TTP PET+ HR 1.76 (1.2-2.4; p=0.003), OS PET+ HR 1.8 (1.2-2.6; p=.0016).
Conclusions: MM is a heterogeneous disease that relies on different assessments for determining response, activity, and prognosis. Among these, PET/CT has shown promise in predicting prognosis, independent of disease state and conventional prognostic factors. Our study confirms these findings, demonstrating that an abnormal PET/CT near day 100 post-transplant is predictive of shorter TTP and OS. This prognostic significance remained even after adjusting for disease response (CR vs. non-CR), which could be explained by the sometimes patchy distribution of myeloma in the bone marrow, and identification of extramedullary disease by PET/CT. Taken together, these results demonstrate a possible role for incorporating PET/CT in the post-transplant setting as an independent prognostic variable in stratifying disease risk.
Dingli:Apellis: Consultancy; Janssen: Consultancy; Rigel: Consultancy; Millenium: Consultancy; Karyopharm Therapeutics: Research Funding; Sanofi-Genzyme: Consultancy; Bristol Myers Squibb: Research Funding; Alexion: Consultancy. Gertz:NCI SPORE MM: Research Funding; International Waldenstrom Foundation: Research Funding; Amyloidosis Foundation: Research Funding; Springer Publishing: Patents & Royalties; i3Health: Consultancy; Advisory Board for Proclara: Membership on an entity's Board of Directors or advisory committees; Advisory Board for Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; DAVA oncology: Speakers Bureau; Johnson and Johnson: Speakers Bureau; Celgene: Consultancy; Data Safety Monitoring board from Abbvie: Membership on an entity's Board of Directors or advisory committees; Physicians Education Resource: Consultancy; Medscape: Consultancy, Speakers Bureau; Amgen: Consultancy; Appellis: Consultancy; Annexon: Consultancy; Spectrum: Consultancy, Research Funding; Janssen: Consultancy; Sanofi: Consultancy; Prothena: Consultancy; Alnylam: Consultancy; Ionis/Akcea: Consultancy. Dispenzieri:Janssen: Research Funding; Celgene: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Intellia: Research Funding; Takeda: Research Funding. Kapoor:Takeda: Honoraria, Research Funding; Amgen: Research Funding; Sanofi: Consultancy, Research Funding; Janssen: Research Funding; Celgene: Honoraria; Cellectar: Consultancy; GlaxoSmithKline: Research Funding. Lin:Sorrento: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vineti: Consultancy; Novartis: Consultancy; Celgene: Consultancy, Research Funding; Bluebird Bio: Consultancy, Research Funding; Juno: Consultancy; Legend BioTech: Consultancy; Merck: Research Funding; Takeda: Research Funding; Gamida Cells: Consultancy; Janssen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding. Kumar:Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Merck: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Dr. Reddy's Laboratories: Honoraria; Novartis: Research Funding; Kite Pharma: Consultancy, Research Funding; Karyopharm: Consultancy; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Cellectar: Other; Tenebio: Other, Research Funding; Sanofi: Research Funding; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Carsgen: Other, Research Funding; Genecentrix: Consultancy; MedImmune: Research Funding; BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.